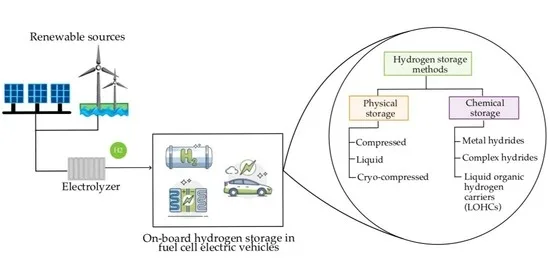
Introduction
In the quest for sustainable and renewable energy sources, the Proton Exchange Membrane (PEM) electrolyzer has emerged as a pivotal technology. This device plays a crucial role in the production of green hydrogen, an essential component in the push towards clean energy. This article explores the science behind PEM electrolyzers, their working principles, benefits, and potential impact on the future of energy.
Understanding PEM Electrolyzers
A PEM electrolyzer is a device that uses electricity to split water into hydrogen and oxygen, a process known as electrolysis. The PEM electrolyzer is distinct for its use of a solid polymer electrolyte and its efficient operation at relatively low temperatures.
The Core Components
- Anode and Cathode: The two pem electrolyzer where the reactions occur.
- Proton Exchange Membrane: A solid polymer electrolyte that only allows protons to pass through.
- Catalysts: Typically platinum or iridium-based, these are used to increase the efficiency of the electrolysis reaction.
The Electrolysis Process
- Water Supply: Water is supplied to the anode side of the electrolyzer.
- Electrical Input: When electricity is applied, water molecules at the anode are split into oxygen, protons (hydrogen ions), and electrons.
- Proton Transfer: The PEM allows only the protons to pass through to the cathode side, while electrons travel around the circuit, creating an electric current.
- Hydrogen Production: At the cathode, protons and electrons recombine to form hydrogen gas.
- Oxygen Release: Oxygen is released at the anode as a by-product.
Benefits of PEM Electrolyzers
Efficiency and Purity
PEM electrolyzers are known for their high efficiency in converting electricity to hydrogen. They also produce highly pure hydrogen, crucial for various applications, including fuel cells.
Compact and Scalable
Due to their solid-state design, PEM electrolyzers are compact and can be scaled easily, making them suitable for various applications, from small-scale production to large industrial plants.
Flexibility and Response Time
These electrolyzers can operate efficiently under varying loads, making them compatible with intermittent renewable energy sources like solar or wind power. They also have a rapid response time to changes in electrical input.
Challenges and Future Potential
Material Costs and Durability
The high cost of catalyst materials like platinum and the durability of the membrane remain challenges. Ongoing research focuses on finding cost-effective and robust alternatives.
Energy Source for Electrolysis
The overall environmental impact of hydrogen production depends on the source of electricity for electrolysis. Using renewable energy sources enhances the green credentials of hydrogen production.
Integrating with Renewable Energy Systems
PEM electrolyzers are well-suited to pair with renewable energy systems, facilitating the storage of excess energy and contributing to grid stabilization.
Conclusion
PEM electrolyzers represent a critical technology in the advancement of clean energy solutions. Their ability to efficiently produce pure hydrogen, coupled with their compatibility with renewable energy sources, positions them as a key player in the transition to a sustainable energy future. As technology continues to evolve and overcome current challenges, PEM electrolyzers are set to play a pivotal role in the global pursuit of a cleaner, greener, and more sustainable world.